by
John R. Fischer, Senior Reporter | March 27, 2019
Volpara Solutions has expanded its existing relationship with GE Healthcare by making the tech giant the global distributor of its VolparaDensity software.
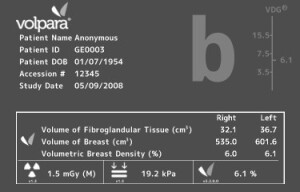
In use in more than 35 countries, VolparaDensity utilizes machine learning to analyze mammograms and provide automated, objective, and volumetric breast density assessments to help radiologists determine if patients require supplemental testing to confirm the presence of breast cancer. The expansion extends its use to GE customers outside of the U.S.
“Volpara Solutions and GE Healthcare have been partnering in the United States since 2015. Now GE customers outside of the United States can also purchase VolparaDensity bundled with GE's breast imaging modalities such as the Senographe Pristina and the Invenia Automated Breast Ultrasound (ABUS),” Dave Mezzoprete, director, business development of Volpara Solutions, told HCB News. “Volpara and GE can provide truly comprehensive dense breast screening.”
Around 40-50 percent of women in the U.S. possess dense breast tissue, a condition which raises the risk of developing breast cancer in one’s lifetime, and can obscure the presence of tumors and prevent them from being detected during routine 2D and 3D mammograms. Awareness of the risks of dense breast tissue is growing, as evidenced by the recent
passage in the U.S. of a law requiring all mammography reports to include up-to-date information on the density of a patient’s breast tissue.
The use of advanced screening options, such as breast MR and breast ultrasound, including GE’s ABUS system, can more easily pick up on breast cancer, as evidenced, according to Mezzoprete, by the initial findings from a Dutch trial presented at ECR, which indicate a dramatic drop in the number of interval cancers for women with high volumetric breast density when subjected to these forms of imaging.
Designed to assign a breast density category in correlation with the fourth and fifth editions of the BI-RADS model, another study in 2018 found the clinical application
to be on equal footing with the subjective evaluations of radiologists in accurately predicting a woman’s risk of developing breast cancer and the risk of interval cancers. Women with extremely dense breasts who were assessed with the system had a 5.65 times higher risk of interval cancer and a 1.43 times higher risk of screen-detected cancer than women with scattered fibroglandular densities.
The system is backed by more than 100 peer-reviewed papers in all, and more than 250 publications.
“The success and validation of VolparaDensity, with more than 100 peer-reviewed published papers, provides strong clinical support for dense breast screening and is an important driver of density awareness among clinicians,” said Mezzoprete. “When bundled together, VolparaDensity and the GE imaging modalities are ideal for dense breast screening. Facilities have access to a simple solution for ensuring that patients receive optimal screening when the sensitivity of mammography alone is too low.”
The system is FDA cleared and CE marked, and approved for use by Health Canada and Australia’s Therapeutic Goods Administration.