FDA clears RFPi's iCertainty blood flow and perfusion imaging solution
by
John R. Fischer, Senior Reporter | January 21, 2019
iCertainty
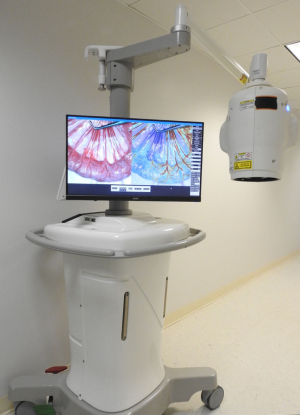
The FDA has given clearance to the iCertainty blood flow and perfusion imaging medical device developed by RFPi for surgeons.
Designed to depict real-time blood flow and perfusion in vascular structures and critical tissues during surgery, the system cuts down procedural complications and repeat surgeries, and requires no injections, dyes, radiation, direct patient contact or interruptions in procedures.
"Documenting optimal blood flow and perfusion to critical vascular structures and tissues is vital to surgical procedures. But today, surgeons are unable to see or measure blood flow in tissues in real time, forcing them to make technical decisions without complete information," Jeffery Basham, CEO of RFPi, told HCB News. "RFPi’s iCertainty instantly delivers non-invasive visualization of blood flow and tissue perfusion—and without any dyes, injections, patient contact or ionizing radiation. Immediate visualization in terms of both capturing an image and analyzing it allows surgeons to adjust their approach and make medical decisions in real time."
Existing modalities such as ICG injection, X-ray, ultrasound, CT, MR or PET scans lack the ability to provide real-time images and data about blood flow and tissue perfusion without interrupting the procedure, requiring patient contact, and increasing patient risk.
In addition to reduced procedural complications and repeat procedures, iCertainty offers reduced hospital costs, and objectively measures and helps to improve clinical outcomes.
The source behinds it real-time visualization and quantification of blow flow and perfusion is its multi-spectral physiologic visualization (MSVP), a technology consisting of low-energy lasers, high-speed imaging cameras, proprietary analysis techniques and flow-calculation algorithms.
Using a federal grant as funding, researchers at RFPi are studying the ability of MSPV to accurately monitor a patient’s basic cardiovascular parameters at the point of care without the patient coming into physical contact with staff members, and then transfer the information to other healthcare providers. Such a capability would be a strong asset in trauma-care centers and for teams on the battlefield.
"RFPi anticipates that peripheral vascular, gastrointestinal, and plastic surgeons will be among the first adopters of its technology," said Basham. "That being said, MSPV offers broad possible uses in the field of medicine. RFPi’s technology is relevant to all medical disciplines where real-time imaging and analysis of the physiology and pathophysiology of blood flow and tissue perfusion affects a clinician’s technical decisions as well as clinical outcomes."
Another solution in the works by RFPi is a mobile device for outpatient settings that would particularly benefit wound care and diabetic health, a condition on the rise throughout America. The initial design work on this form factor is funded through a loan from North Carolina Biotechnology Center.
iCertainty has been cleared by the FDA for imaging blood flow and perfusion in tissue up to a depth of 4-5 mm. Initial applications are expected to involve gastrointestinal and plastic surgeries and lower-leg vascular procedures.
RFPi is currently focused on securing Series B financing to commercialize iCertainty, having closed its Series A round funding in May 2017, and market it to surgeons and medical centers.
|
|
|
You Must Be Logged In To Post A Comment
|
