MARS imaging and the arrival of 3D color X-rays
November 28, 2018
By Hannah Prebble
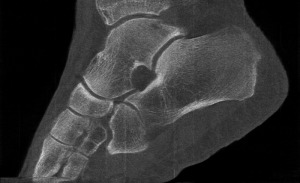
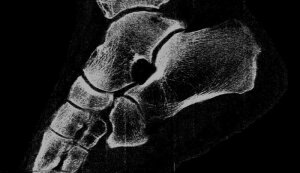
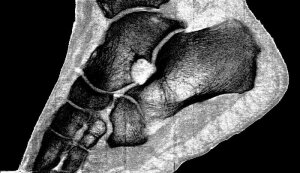
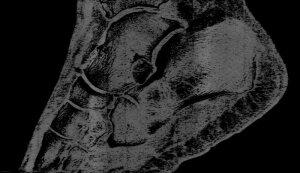
Professor Phil Butler is now the proud owner of one of the world’s most famous ankles.
Images of his ankle and wrist have been viewed by over 50 million people. The astonishing detail in the images has come from major advancements in the medical imaging field. By moving to a smaller pixel size and measuring the energy of photons, by counting at 8 energy thresholds, the MARS scanner is capable of generating images that contain over 8000 times more information than standard human-scale CT.
In addition to being the first person scanned by the MARS scanner, Butler is one of the leading scientists behind its development and the CEO of MARS Bioimaging. Although his background is academic research, the University of Canterbury physicist has been described by some as a serial researcher entrepreneur, having amongst other startups previously commercialized a novel laser for the treatment of port wine stain birthmarks.
In developing the MARS scanner, Butler successfully brought together his diverse interests, which include pure mathematics, high-energy physics and medical imaging technology. “I have always been motivated to take the results of physics research to people who can use it,” he said. “With MARS we are taking some innovative detectors from high-energy physics at CERN and creating a device which will improve medical diagnosis for millions of people. The thought inspires me and helps with long slog required.”
Despite the immediacy of the announcement, the actual machine has been many years in the making. It was Butler’s initial involvement in the CERN CMS collaboration, and subsequently the Medipix3 collaboration, which brought the detector technology and the idea for the MARS scanner to New Zealand over a decade ago.
The idea of using the color of X-rays for medical imaging originated in the 1970s with several theory papers proposing that it might be useful to capture the energy information contained within the X-ray photons. By 1995, CERN had begun to develop semiconductor, direct-conversion detectors for high-energy particle physics experiments.
It was realized these detectors could achieve the kind of imaging proposed in those original theory papers. After Butler joined the CERN CMS team in 2002, he saw that New Zealand had the skills to develop a medical scanner using the Medipix3 detector. By 2006, Phil was joined by his radiologist son Anthony, and together with others, they performed the first MARS scans using a bench top prototype system.
On the back of the successful prototype, MARS Bioimaging Ltd. formed, with the goal of creating a commercial preclinical system, and scaling the technology for clinical use. “Modern imaging technologies – ultrasound, MR, CT and PET – were incrementally improving medical diagnosis year by year, but there were still many common diseases that couldn’t be diagnosed,” he said, “…and still cannot.”
Developing and marketing a preclinical system was a conscious decision to introduce a high-resolution color X-ray imaging system to researchers first, giving them a head start toward addressing current imaging challenges and answering the question of how this type of quantitative imaging can be used to go beyond current clinical imaging techniques.
The technology has come a long way in the past decade. “In the early days, a mouse took around 24 hours to scan and over a week to reconstruct,” said Butler. “Today, a mouse can be scanned in a matter of minutes, and reconstructed in an hour.”
The first challenge has always been to collect the best energy information possible. The second challenge is to best use that information to analyze the object. Then you need to produce images that convey that information to users.
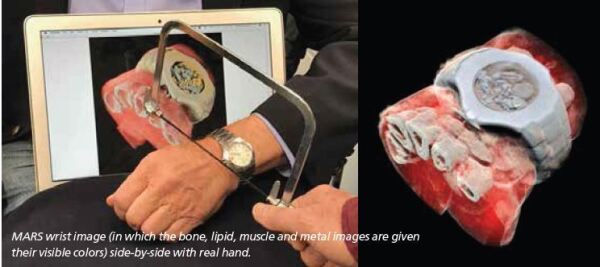
The energy information and the small pixel size enable MARS to produce lifelike images such as the image of Phil’s watch and half his hand, an image that went viral with tens of millions of reposts. Phil remarked, “I find the image beautiful, even though some find it horrid.”
“My turning ankle is less horrid perhaps, but contains more information, particularly if you seek out the high resolution images of the calcium, fatty, and water maps. We get exquisite separation of materials at the 80 micron, or 0.08 mm, scale.”
Looking to the future, MARS imaging has already demonstrated its usefulness for orthopedic joint scanning, and it is easy to see that within a few years, head and neck scanning will be viable. MARS scans of shoulders and hips will require methods to analyze and display terabyte data sets.
The preclinical research has demonstrated how transformative this technology will be in areas such as cancer detection, bone health, bone-metal interface imaging, and cardiovascular health assessment.
“The key to the technology is being able to achieve much higher-resolution images than traditional modalities and simultaneously receive information about the material composition of the object,” said Butler. “In scaling the technology to wrists and ankles, the goal was to maintain that same resolution and diagnostic quality that the small system had already demonstrated.”
There is clearly a need for new clinical imaging modalities. Since the wrist and ankle images were published, there has been a flood of requests from people for whom traditional imaging hasn’t provided the diagnostic information they need. MARS is an interesting modality as it overlaps across both CT- and MR-type imaging. It is capable of scanning both bone and soft tissue, and can also image through and around metal. This ability to image with metal is particularly important for assessing bone healing after a joint replacement, a procedure that is set to double in the next few years.
The biggest challenge now is to get the MARS imaging technology to the people who need it – clinicians and patients. There are the usual challenges of getting a product from prototype through to FDA approval.
One drawback of the MARS images is the amount of information generated with each scan. The extra information is beneficial for disease detection and diagnosis, but it can be a challenge to deal with. For the time being, the team is focusing on developing products for imaging smaller body parts like wrists or necks.
In time, data processing techniques should catch up and allow a whole body scan. “The first human trials will be used to demonstrate some of the applications of MARS scanning that have already been researched using our preclinical system, for example, looking at early biochemical changes in cartilage,” said Butler.
For him, the most surprising thing to come out of this project has been how well the raft of skills from a multinational, interdisciplinary team complement each other. Over 50 people including physicists, clinicians, scientists and engineers, are currently working on the commercialization of the scanner and research into clinical applications. According to Butler, “It is this interdisciplinary approach which has driven the success of the project, and allowed an innovation from New Zealand to make waves on a global scale.”
About the author: Hannah Prebble is a clinical applications researcher for MARS Bioimaging Ltd. in Christchurch, New Zealand.
Professor Phil Butler is now the proud owner of one of the world’s most famous ankles.
Images of his ankle and wrist have been viewed by over 50 million people. The astonishing detail in the images has come from major advancements in the medical imaging field. By moving to a smaller pixel size and measuring the energy of photons, by counting at 8 energy thresholds, the MARS scanner is capable of generating images that contain over 8000 times more information than standard human-scale CT.
In addition to being the first person scanned by the MARS scanner, Butler is one of the leading scientists behind its development and the CEO of MARS Bioimaging. Although his background is academic research, the University of Canterbury physicist has been described by some as a serial researcher entrepreneur, having amongst other startups previously commercialized a novel laser for the treatment of port wine stain birthmarks.
In developing the MARS scanner, Butler successfully brought together his diverse interests, which include pure mathematics, high-energy physics and medical imaging technology. “I have always been motivated to take the results of physics research to people who can use it,” he said. “With MARS we are taking some innovative detectors from high-energy physics at CERN and creating a device which will improve medical diagnosis for millions of people. The thought inspires me and helps with long slog required.”
Despite the immediacy of the announcement, the actual machine has been many years in the making. It was Butler’s initial involvement in the CERN CMS collaboration, and subsequently the Medipix3 collaboration, which brought the detector technology and the idea for the MARS scanner to New Zealand over a decade ago.
The idea of using the color of X-rays for medical imaging originated in the 1970s with several theory papers proposing that it might be useful to capture the energy information contained within the X-ray photons. By 1995, CERN had begun to develop semiconductor, direct-conversion detectors for high-energy particle physics experiments.
It was realized these detectors could achieve the kind of imaging proposed in those original theory papers. After Butler joined the CERN CMS team in 2002, he saw that New Zealand had the skills to develop a medical scanner using the Medipix3 detector. By 2006, Phil was joined by his radiologist son Anthony, and together with others, they performed the first MARS scans using a bench top prototype system.
On the back of the successful prototype, MARS Bioimaging Ltd. formed, with the goal of creating a commercial preclinical system, and scaling the technology for clinical use. “Modern imaging technologies – ultrasound, MR, CT and PET – were incrementally improving medical diagnosis year by year, but there were still many common diseases that couldn’t be diagnosed,” he said, “…and still cannot.”
Developing and marketing a preclinical system was a conscious decision to introduce a high-resolution color X-ray imaging system to researchers first, giving them a head start toward addressing current imaging challenges and answering the question of how this type of quantitative imaging can be used to go beyond current clinical imaging techniques.
The technology has come a long way in the past decade. “In the early days, a mouse took around 24 hours to scan and over a week to reconstruct,” said Butler. “Today, a mouse can be scanned in a matter of minutes, and reconstructed in an hour.”
The first challenge has always been to collect the best energy information possible. The second challenge is to best use that information to analyze the object. Then you need to produce images that convey that information to users.
The energy information and the small pixel size enable MARS to produce lifelike images such as the image of Phil’s watch and half his hand, an image that went viral with tens of millions of reposts. Phil remarked, “I find the image beautiful, even though some find it horrid.”
“My turning ankle is less horrid perhaps, but contains more information, particularly if you seek out the high resolution images of the calcium, fatty, and water maps. We get exquisite separation of materials at the 80 micron, or 0.08 mm, scale.”
Looking to the future, MARS imaging has already demonstrated its usefulness for orthopedic joint scanning, and it is easy to see that within a few years, head and neck scanning will be viable. MARS scans of shoulders and hips will require methods to analyze and display terabyte data sets.
The preclinical research has demonstrated how transformative this technology will be in areas such as cancer detection, bone health, bone-metal interface imaging, and cardiovascular health assessment.
“The key to the technology is being able to achieve much higher-resolution images than traditional modalities and simultaneously receive information about the material composition of the object,” said Butler. “In scaling the technology to wrists and ankles, the goal was to maintain that same resolution and diagnostic quality that the small system had already demonstrated.”
There is clearly a need for new clinical imaging modalities. Since the wrist and ankle images were published, there has been a flood of requests from people for whom traditional imaging hasn’t provided the diagnostic information they need. MARS is an interesting modality as it overlaps across both CT- and MR-type imaging. It is capable of scanning both bone and soft tissue, and can also image through and around metal. This ability to image with metal is particularly important for assessing bone healing after a joint replacement, a procedure that is set to double in the next few years.
The biggest challenge now is to get the MARS imaging technology to the people who need it – clinicians and patients. There are the usual challenges of getting a product from prototype through to FDA approval.
One drawback of the MARS images is the amount of information generated with each scan. The extra information is beneficial for disease detection and diagnosis, but it can be a challenge to deal with. For the time being, the team is focusing on developing products for imaging smaller body parts like wrists or necks.
In time, data processing techniques should catch up and allow a whole body scan. “The first human trials will be used to demonstrate some of the applications of MARS scanning that have already been researched using our preclinical system, for example, looking at early biochemical changes in cartilage,” said Butler.
For him, the most surprising thing to come out of this project has been how well the raft of skills from a multinational, interdisciplinary team complement each other. Over 50 people including physicists, clinicians, scientists and engineers, are currently working on the commercialization of the scanner and research into clinical applications. According to Butler, “It is this interdisciplinary approach which has driven the success of the project, and allowed an innovation from New Zealand to make waves on a global scale.”
About the author: Hannah Prebble is a clinical applications researcher for MARS Bioimaging Ltd. in Christchurch, New Zealand.