AI to workflow, breast imaging makes advances
July 09, 2018
by Lisa Chamoff, Contributing Reporter
With facilities continuing to adopt digital breast tomosynthesis, manufacturers are expanding system features and software providers are harnessing deep learning to help radiologists read images more efficiently.
At the same time, companies are releasing new specimen imaging devices to aid surgeons, along with new MR and breast ultrasound systems.
Here’s an overview of what’s new from more than a dozen companies:
AGFA HealthCare
AGFA has made more than 50 changes to its Enterprise Imaging platform in the past year, just focused on breast imaging and improving the user experience and workflow. The changes permit customers to work smarter and faster despite the increasing sophistication of clinical demands, said Bob Craske, the director of enterprise imaging, radiology and clinical applications for AGFA HealthCare.
Included in the key changes are the ability to toggle between 2D and 3D within a viewport, the ability to use a third monitor for ultrasound and MR images and tools that allow users to easily drag and drop digital breast tomosynthesis 2D data into a viewport with 3D functionality for rapid image processing. These changes deliver a much smoother and easier workflow to navigate complex studies, Craske said.
AGFA has worked closely with its customers, both academic and non-academic facilities, to make the improvements, Craske said.
"There are a lot of nuances in breast imaging that can make CT and MR look like child's play, even as complex as they can be,” Craske said. "We added simplified navigation tools for breast imaging radiologists, to make it easier for them to adjust their display, their visualization. Even something as simple as one less mouse click. We're continuously adding new tools for productivity and clinical efficiency. Coupling these functional changes with the rules-based workflow engine employed by Enterprise Imaging brings a new and positive dynamic to the market.”
Carestream
Carestream recently released customized user preferences for its Vue Mammo Workstation.
Thierry Verstraete, Carestream’s worldwide product line manager for healthcare IT clinical solutions and analytics, said the changes follow the shift to value-based care.
“We worked a lot on the overall user experience of the product,” Verstraete said.
For example, if a radiologist has specific preferences in a display protocol for specific imaging modalities, for example, they can apply the preferences in their user settings.
“For my user profile, if I want the zoom factor to be 120 percent or 150 percent, that is a time saver because now it’s there as a default,” Verstraete said.
There is also the ability to set slab thickness preference for tomosynthesis to better identify lesions.
Working with partners, Carestream released a Workflow Orchestrator that uses algorithms to prioritize workflow. Customers can purchase the module, which is not specific to mammography, separately.
In the future, Carestream is looking to offer more support for automated breast ultrasound and automated breast volume scanner (ABVS) with their own diagnostic viewers.
“At the moment, if a customer wants it, we can accomplish it with desktop integration,” Verstraete said. “It ties back to one workstation philosophy.”
Dilon Technologies
Dilon, which manufactures molecular breast imaging systems, recently released a biopsy accessory for its Discovery NM750b dual head MBI system.
Pjerin Luli, vice president of U.S. sales and clinical education at Dilon, said the MBI biopsy system can locate lesions that are not detected with other modalities.
“How do you biopsy lesions not seen on ultrasound?” Luli said.
Luli said Dilon’s MBI product stands out from the competition by offering the ability to perform biopsy and also using air to cool its detectors instead of water. The system also offers a detector with a larger field of view, Luli said.
The company is planning its first installation of its first MBI system with biopsy in July 2018.
Faxitron
In May, Faxitron’s Vision CT became the first FDA-approved clinical CT system 3D specimen radiography, according to the company.
The Vision CT is designed to provide easily navigable, true 3D data and rendering of the specimen, in addition to immediate traditional orthogonal images, said Donogh O’Driscoll, Faxitron’s chief operating officer. As a result, it enables enhanced margin assessment and drives the potential for improved surgical outcomes.
The compact mobile system is used in the operating room for quick excised specimen margin assessment during surgery. Wait times when sending a specimen to radiology can be 15 to 30 minutes during surgery, O’Driscoll said.
O’Driscoll said the company “brought specimens into the 3D world” with the launch of the VisionCT. The system can reconstruct the whole specimen volume — up to 512 frames — in under four minutes in addition to providing traditional orthogonal images in under 30 seconds, which means surgeons can move through the specimen in slices, with the goal of reducing re-incision rates and callbacks, according to O’Driscoll.
“They can interrogate the specimen to a far deeper level,” O’Driscoll said. “It’s far better to provide as much detail as possible and put that in the hands of physicians to make the ultimate call for better patient outcomes.
“Abnormalities in breast tissue are often caught at earlier stages now, thanks to advancements in imaging and breast screening. So there is a parallel need for ongoing advancements when assessing and confirming margin status during surgery,” O’Driscoll said. “With VisionCT, providers can now obtain a clearer, more detailed view of breast specimen margin status than ever before, helping to achieve better surgical outcomes. For decades we’ve been committed to pioneering new technologies that contribute to a higher standard of care for patients and we are proud that our legacy continues with the launch of this innovative new system.”
Fujifilm
Fujifilm introduced two products last year. In January 2017, it upgraded the ASPIRE Cristalle, introduced in 2013, to include digital breast tomosynthesis, and at the end of 2017, the company released mobile tomosynthesis.
“Even for women who do have insurance, only 68 percent of them have had a recent mammogram,” said Susan Crennan, women’s health product marketing manager for FUJIFILM Medical Systems U.S.A., Inc. “We want to make sure all women have access to the latest technologies.”
The company works with a range of mobile imaging vendors for the exams.
The ASPIRE Cristalle has an amorphous selenium detector with Hexagonal Close Pattern (HCP) capture circuitry design, which Crennan said outperforms traditional pixel arrays with a 20 percent increase in detector sensitivity. With 50 microns of resolution, the tomosynthesis exposure time on the ASPIRE Cristalle is four seconds or less, meaning a low likelihood of patient movement, Crennan said. There is also a 15-second interval between subsequent images.
There’s no need to wait for the machine and technologist,” Crennan said. “Other systems tend to have longer intervals — up to 40 seconds — between exposures.”
This year, the company released Dynamic Visualization II for Mammography (DVIIm). The second generation of the software offers intelligent auto recognition of breast tissue, implants and structural characteristics to intelligently adapt image contrast and density, for enhanced diagnostic visibility, according to the company. DVIIm also provides improved contrast and density stability throughout the exposure region and achieves improved visibility across a wide range of breast compositions.
Galen MRI
Galen MRI is currently going through the FDA approval process for its new 1.5-tesla breast MR system, called Emma.
David Tse, the board chairman for Galen MRI, said the abbreviated protocols for Emma reduce the scan time from 45 minutes to 15 minutes. This, in turn, lowers the cost of the scan and helps reduce the cost to around $600 per scan. The AB protocol was tested on 1,500 women at the Memorial Sloan Kettering Imaging Center in New York City.
The system comes with three sizes of breast coils, and the company is in the process of developing a system to reduce noise level. A cloud system, for educational use, will allow the DICOM images to be shared with privacy protection.
There are also machine learning protocols to further reduce the time between the preparatory scan and the proper scan, and two AI systems. The first, called Scan Assistant, scans the images to enhance radiologists' diagnosis, while Report Assistant generates the initial scan report, to facilitate further reporting.
Galen also plans to work with other big data teams, such as Watson's big data team on breast cancer, to further advance the knowledge of breast cancer detection and containment, Tse said.
“With its dedicated coil and AB protocol, Emma will simultaneously help advance early breast cancer detection and lower the cost of breast MR,” Tse said.
GE Healthcare
After the March 2017 debut of its Senographe Pristina 3D Mammography System, GE Healthcare launched the Pristina Dueta, a patient-assisted compression device that the company says can help make the exam less painful.
After the technologist positions the patient, they then give the device to the patient, who can do a final adjustment.
It reduces the patient’s perceived pain levels,” explained Agnes Berzsenyi, president and CEO of GE Healthcare women’s health. They feel empowered and they can be active during the exam. If you pinch yourself, versus someone else pinching you, it’s a big difference.”
In patient studies of the device in Italy and France, four in five of the 160 patients who used the Pristina Dueta said they felt more comfortable during the exam.
In a clinical evaluation of the Dueta used with 30 patients at the Christine E. Lynn Women's Health & Wellness Institute at the Boca Raton Regional Hospital, as part of the FDA submission, medical director Dr. Kathy Schilling, found that patient-assisted compression, compared to compression solely applied by the technologist, produced images of similar quality and didn’t significantly increase the time of the exam.
The technologist sets the compression to the minimum required threshold. The patient can increase compression or they can decrease only once.
Schilling also found that women who were in control of their own compression actually compressed the breast 45 percent more than the technologist did.
In November 2017, GE released the SenoBright HD clinical application. The Contrast Enhanced Spectral Mammography (CESM) exam, available on the Senographe Pristina, can be used as an alternative to breast MR for patients at high risk for breast cancer or who have inconclusive mammogram or ultrasound results.
The IV iodine injection is performed in same room as the mammogram. The exam takes less than seven minutes and the images are available immediately for a radiologist to review.
“It’s a great fit for a facility without access to MR onsite,” Berzsenyi said.
On the software side, GE released Mammo Insights in November 2017. The multivendor software analyzes data using algorithms to gain insight into different types of exams. It looks at dose by breast thickness and composition so facilities can adjust imaging techniques and can compare dose among multiple sites.
“There is a need for understanding of dose,” Berzsenyi said. “We’re starting to see trends where patients are demanding it and they want to know.”
Hologic
Hologic has released several products in the breast imaging space in recent months, with a focus on image quality, improved patient experience and workflow.
The company's 3Dimensions Mammography System, which was FDA cleared in June 2017, has a proprietary feature called the SmartCurve breast stabilization system, which was FDA cleared separately this past March.
The paddle, which mimics the shape of the breast, is designed to deliver a more comfortable experience for the patient, said Tracy Accardi, Hologic's global vice president of research and development, breast and skeletal health solutions.
"The SmartCurve breast stabilization system delivers a more comfortable mammogram without sacrificing image quality," Accardi said.
SmartCurve is also available for previous generations of Hologic mammography systems.
The 3Dimensions system also features Clarity HD high-resolution 3D imaging, which Accardi said provides the highest resolution 3D images in the industry, and can detect subtle lesions, regardless of breast size and density, to pinpoint cancer earlier. Additionally, the Genius 3D Mammography exam, which is available on the 3Dimensions system, reduces callbacks by up to 40 percent compared to 2D imaging.
"The 3Dimensions system is the next generation of 3D imaging," said Accardi of the technology.
In August, Hologic released its Brevera breast biopsy system, with CorLumina imaging technology, in the U.S. It provides real-time imaging and automated post-biopsy specimen handling, negating the need to take samples out to another room for further review.
"The system makes sure you are getting the cancerous tissue you intended to get," Accardi said. "Every eight seconds you can obtain a biopsy sample and view its X-ray. The result is a much quicker workflow for the radiologist, and a faster procedure for the patient."
In May of this year, Hologic released a new portable breast ultrasound system called the Viera portable breast ultrasound system.
The transducer and processing are all handheld and images are transmitted wirelessly to a mobile device or a PACS system.
"The system is the perfect tool for a breast surgeon about to do a biopsy, and makes sure they're looking at the right place in the woman's breast," Accardi said. "They don't have to find and cart in a larger ultrasound system from another room."
The device can also negate the need for wire localization, which would be a win for both a workflow and patient experience perspective, Accardi said.
"Traditionally, portable ultrasound systems have a reputation for compromising on image quality," Accardi said. "However, we've optimized this system to have the best possible image quality and physician workflow. When you're done with the Viera system, you drop it right in your pocket."
In March, Philips announced a partnership with Hologic to combine Hologic's mammography technologies and Philips' portfolio of ultrasound, MR, CT, and X-ray systems, advanced informatics and range of services, including maintenance, upgrades, training and operational performance management services.
In a statement announcing the partnership, Philips said it will allow hospitals easier access to integrated suites of diagnostic imaging modalities, advanced informatics and services for comprehensive breast screening and diagnosis.
iCAD
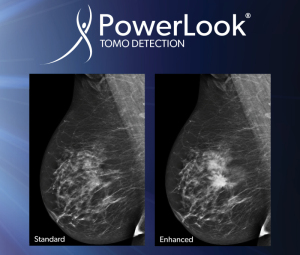
In March 2017, iCAD received FDA approval for PowerLook Tomo Detection, a computer-aided detection solution for breast tomosynthesis that is built on deep learning technology.
The solution is available for use worldwide with GE Healthcare breast tomosynthesis, though the company received CE Mark approval for a new version of the product for use in Europe with Hologic and Siemens systems, as well as those from GE, and looks to having a solution available for other vendors in the future.
Part of the PowerLook Breast Health Solutions platform, PowerLook Tomo Detection identifies suspicious areas in breast tomosynthesis images.
Rodney Hawkins, vice president of marketing for iCAD, said a reader study showed a 29.2 percent reduction in reading time without impacting clinical performance.
Hawkins calls the deep learning technology similar to traditional computer-aided detection methods, “but more advanced.”
Traditional CAD technology “is a little more manual in how the algorithm is taught to look for cancer,” Hawkins said. “A researcher basically guides the algorithm to find cancer. Deep learning is a more advanced subset of machine learning. The computer learns the necessary features itself. We train it on actual cancer cases and it learns on its own what the characteristic features of cancer are. It’s a much more accurate technology. … Similar to 2D CAD, it provides them with a level of confidence, another set of eyes. It’s a lot easier in tomo to miss things because there’s so much information. We’ve heard customers say they’re less fatigued.”
The new product that is currently available in Europe detects soft tissue density and calcifications and provides more information on the certainty of findings, or “how confident the algorithm is,” with a confidence score from zero to 100, Hawkins said.
Ikonopedia
Last year, Ikonopedia released the latest version of its Ikonopedia Breast Reporting and Tracking software.
The new version incorporates a tablet-based questionnaire with the only web-based version of the Tyrer-Cuzick Breast Cancer risk assessment tool utilizing Tyrer-Cuzick version 8, which incorporates density, according to Emily Crane, Ikonopedia's chief executive officer.
Ikonopedia's software also employs what Crane said is the only "closed loop" tracking at a lesion-specific level, which prevents any finding from being lost to follow-up.
In December 2017, the company also released a full EQUIP module to support the new image quality requirements from the FDA.
The EQUIP software gives imaging departments the ability to report, track and address poor image quality at the point of read and also creates randomized audits for review. It then creates all necessary outcome reports.
"They never have to worry about doing a manual audit," Crane said. "We automate all necessary audits for them and they can be printed at the touch of one button."
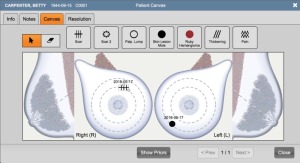
In 2017 Ikonopedia also released Patient Canvas, which gives technologists a visual tool to markup things seen or felt on the outside of the breast — scars, moles, lumps, or pain — so the radiologist might investigate areas with external issues. Patient Canvas also alerts the radiologists when they open the case to any findings on the Patient Canvas, which Crane said enhances technologist and radiologist communication.
IntraMedical Imaging
IntraMedical Imaging recently received CE Mark approval for the Node Seeker 2000, a wireless gamma probe with a tablet-based control unit that can simultaneously find primary tumors based on Iodine 125 (125I) radioactive seed localization and in the sentinel lymph node.
Farhad Daghighian, the chief executive officer of IntraMedical Imaging, said the system, released last year, gives an accurate estimate of the depth of the seed and corrects for the interference of technetium gamma rays while searching for the seed.
“The battery-operated tablet is made for medical use, and by wrapping it in a sterilized jacket, it can be placed on top of the patient in front of the surgeon so the surgeon would not need the nurses to adjust the sound or other parameters,” Daghighian said.
The market for the Node Seeker 2000 is general surgeons, surgical oncologists and nuclear medicine departments.
The Node Seeker 2000 can control gamma ray detection probes and beta probes that IntraMedical manufactures for detection of positron emitting tracers. These PET probes are being used for intraoperative detection of tumors filled by F-18 FDG.
Koning Corporation
Koning Corporation’s Koning Breast CT (KBCT) system was FDA approved in 2015; a redesign reducing the footprint and room size, with input power similar to that of a conventional mammography system, got an FDA nod last November.
The Koning KBCT system removes painful breast compression and is a multi-application device FDA-cleared for both the diagnostic workup as well as a 3D guided biopsy, said David Georges, president of the company’s USA division. It fits into a 10-foot-by-14-foot room, which is similar to rooms designed for stereotactic biopsy.
“Prior to the redesign, the KBCT required the same installation space and input power as a full-body CT,” Georges said. “Doctors could actually proceed right to the 3D guided biopsy after the diagnostic workup if that’s what they choose to do.”
The company claims that with breast compression eliminated, the exam is much more comfortable for the patient.
“We’ve not had a woman out of the thousands of patients we’ve imaged say they’d prefer a mammogram,” Georges said.
With floor space a premium in most facilities, the ability to perform the diagnostic workup and a biopsy in the same room delivers new efficiencies in breast imaging, Georges said.
The technology is based on isotropic imaging of the breast and uses a mammographic X-ray tube and detector to gather true 3D volumetric images in under 10 seconds.
“It means we can visualize areas of interest from any angle equally,” Georges said. “Conventional linear tomography of the breast is a limited sweep angle acquisition and not the way we look at the head, chest and abdomen with whole body CT imaging. Breast tomosynthesis is pseudo 3D, whereas Koning is introducing true volumetric 3D of the breast.
“What physicians are telling us [is that] they finally have images that are free from distortion from compression,” Georges said. “Tissue is free from overlapping structures, a known cause of missed cancers in mammography. Until you separate structures, you will never have a clear view of what is cancer and what is not cancer.”
Georges said the dose is in the same range as dose from a diagnostic mammogram and equal to or less than digital breast tomosynthesis for a diagnostic workup. As with all imaging, dose is dependent on the size and thickness of the breast.
“The next challenge for us is to design a smaller device that will deliver low-dose screening exams integrating our true isotropic 3D acquisition process to obtain clear unobstructed images of early stage breast cancers before they spread,” Georges said. “We’re very close to achieving this goal.”
The company has 12 installations, two of which are the new design — one in Knoxville, Tennessee and one in Bangkok, Thailand.
There is currently a study of contrast-enhanced breast CT at the University of Rochester, where research for the device originally began. “Similar to whole body CT with and without contrast, we anticipate significant advantages by applying contrast-enhanced breast CT imaging where applicable,” Georges said.
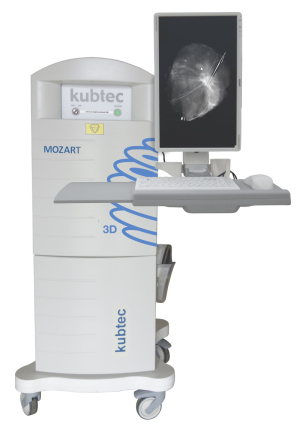
Kubtec
Kubtec’s MOZART System, an intraoperative tomosynthesis scanner designed to be used during lumpectomies, received an upgrade recently with the implementation of voice control and augmented intelligence.
The voice control feature allows surgeons to operate the system using simple commands — generated by focus groups of surgeons — without breaking the sterile field.
“They can give commands to the instrument itself,” said John Leach, vice president of marketing for Kubtec. "They can run any 3-D scans, zooms or highlight areas of interest."
Surgeons have said they appreciate the fact that they don’t have to break scrub or direct OR staff to use the machine.
“It doesn’t talk back,” Leach joked.
The new augmented intelligence automatically identifies the lesion in the image and can show the surgeon whether they’ve removed the entire tumor.
The features are available as a separate package for the MOZART called the AI package, released in May at the American Society of Breast Surgeons annual meeting in Orlando. It is also packaged with new systems.
"We believe the space used in the OR is too valuable to be used to produce just an image,” Leach said. “Our focus is on enhancing the physician's ability to get the information they need from the image in order to make rapid, informed intraoperative decisions that benefit patient outcomes.”
QView Medical
In December, QView Medical received FDA PMA approval for its QVCAD solution for use with the GE Invenia 3D ABUS automated breast ultrasound system. In March 2017, it received the CE Mark for use of the product with Siemens as well as GE.
The AI product is used for screening asymptomatic patients with dense breasts after a negative mammogram.
CAD for mammography has become the standard of care, so it was natural for the company to develop a similar product, using deep learning for automated breast ultrasound exams, said Bob Foley, vice president of regulatory affairs and marketing strategy for QView Medical. Each ABUS exam leads to images with 2,000 slices, with a read time of five to eight minutes.
“We recognized there was a need to be able to read more productively,” Foley said.
Foley said the QVCAD is able to reduce reading time to approximately two minutes without compromising diagnostic accuracy.
Screenpoint Medical
This Netherlands-based company recently released Transpara, an AI product that helps radiologists read mammograms and digital breast tomosynthesis, for use in Europe.
Nico Karssemeijer, the chief executive officer of Screenpoint Medical, said Transpara is different from CAD in that it provides more than a guide to the images.
“CAD systems are very limited because they only point out regions for radiologists to look at,” Karssemeijer said. “Our system interprets abnormalities and provides a cancer suspiciousness score for the whole mammogram that can be used as a second opinion. With the current release, we are reading mammograms as good as experienced radiologists.”
Karssemeijer stressed that the product is not intended to replace radiologists, noting that in the European market, two radiologists are required to read a mammogram.
“This is intended to partly replace one of the two,” Karssemeijer said. “There are some countries where there is a big shortage of radiologists. It’s only going to become more difficult with breast tomosynthesis. This is one of the big problems we have to solve before tomo is used for breast screening programs in Europe.”
The company is in the process of getting FDA clearance for Transpara and is currently doing clinical studies in the U.S.
Siemens Healthineers
In March, Siemens Healthineers received FDA clearance for its MAMMOMAT Revelation, which was shown at RSNA 2017.
The premium mammography system features an integrated specimen imaging tool called InSpect, as well as a 3D tomo breast biopsy solution, the ability to perform contrast-enhanced mammography, and automated breast density measurement at the point of care.
“This option can save time, reduce costs and minimize the anxiety of being called back, especially where a woman might have to wait for an MR,” said Pam Cumming, senior director of product marketing for women's health at Siemens Healthineers North America. “It does give another nice option for people who don’t have easy access to MR.”
The MAMMOMAT Revelation also builds on the previous generation system, the MAMMOMAT Inspiration, with a wide, 50-degree field of view that allows better separation of overlapping breast tissue, resulting in improved visualization of subtle abnormalities, Cumming said.
“Overlapping tissue is a problem in mammography,” said Cumming, who noted that the depth resolution is 3.5 times higher than that of their competitors’ systems.
Cumming also noted that the wide angle might one day allow for reduced compression.
“Clinical data is saying you might not need to do as much compression,” Cumming said. “It’s the power of the wide angle that’s creating the basis for conversation and what might be possible.”
The MAMMOMAT Revelation also performs automated breast density measurement during the mammogram, instead of after the patient has left the imaging facility.
Volpara
Volpara recently released a new software feature that provides feedback on how well the technologist did positioning the patient, by assigning mammographic studies into perfect, good, moderate and inadequate categories.
Training videos, which feature Louise Miller, co-founder of Mammography Educators, are embedded in the company's VolparaEnterprise software and will be based on exam statistics.
The software performs an automated assessment of positioning on every mammogram and will note, for example, if the technologist is not getting inframammary folds into the image, said Julian Marshall, Volpara’s chief product officer.
Since the feature is a quality control device, it doesn’t require FDA clearance.
The software, with cost depending on how big the facility is and how many mammograms it does, is designed to reduce the number of technical recalls, which facilities don’t receive reimbursement for.
“Our key products are being adopted by large health systems because they see it increasing quality across the system,” Marshall said.
At the same time, companies are releasing new specimen imaging devices to aid surgeons, along with new MR and breast ultrasound systems.
Here’s an overview of what’s new from more than a dozen companies:
AGFA HealthCare
AGFA has made more than 50 changes to its Enterprise Imaging platform in the past year, just focused on breast imaging and improving the user experience and workflow. The changes permit customers to work smarter and faster despite the increasing sophistication of clinical demands, said Bob Craske, the director of enterprise imaging, radiology and clinical applications for AGFA HealthCare.
Included in the key changes are the ability to toggle between 2D and 3D within a viewport, the ability to use a third monitor for ultrasound and MR images and tools that allow users to easily drag and drop digital breast tomosynthesis 2D data into a viewport with 3D functionality for rapid image processing. These changes deliver a much smoother and easier workflow to navigate complex studies, Craske said.
AGFA has worked closely with its customers, both academic and non-academic facilities, to make the improvements, Craske said.
"There are a lot of nuances in breast imaging that can make CT and MR look like child's play, even as complex as they can be,” Craske said. "We added simplified navigation tools for breast imaging radiologists, to make it easier for them to adjust their display, their visualization. Even something as simple as one less mouse click. We're continuously adding new tools for productivity and clinical efficiency. Coupling these functional changes with the rules-based workflow engine employed by Enterprise Imaging brings a new and positive dynamic to the market.”
Carestream
Carestream recently released customized user preferences for its Vue Mammo Workstation.
Thierry Verstraete, Carestream’s worldwide product line manager for healthcare IT clinical solutions and analytics, said the changes follow the shift to value-based care.
“We worked a lot on the overall user experience of the product,” Verstraete said.
For example, if a radiologist has specific preferences in a display protocol for specific imaging modalities, for example, they can apply the preferences in their user settings.
“For my user profile, if I want the zoom factor to be 120 percent or 150 percent, that is a time saver because now it’s there as a default,” Verstraete said.
There is also the ability to set slab thickness preference for tomosynthesis to better identify lesions.
Working with partners, Carestream released a Workflow Orchestrator that uses algorithms to prioritize workflow. Customers can purchase the module, which is not specific to mammography, separately.
In the future, Carestream is looking to offer more support for automated breast ultrasound and automated breast volume scanner (ABVS) with their own diagnostic viewers.
“At the moment, if a customer wants it, we can accomplish it with desktop integration,” Verstraete said. “It ties back to one workstation philosophy.”
Dilon Technologies
Dilon, which manufactures molecular breast imaging systems, recently released a biopsy accessory for its Discovery NM750b dual head MBI system.
Pjerin Luli, vice president of U.S. sales and clinical education at Dilon, said the MBI biopsy system can locate lesions that are not detected with other modalities.
“How do you biopsy lesions not seen on ultrasound?” Luli said.
Luli said Dilon’s MBI product stands out from the competition by offering the ability to perform biopsy and also using air to cool its detectors instead of water. The system also offers a detector with a larger field of view, Luli said.
The company is planning its first installation of its first MBI system with biopsy in July 2018.
Faxitron
In May, Faxitron’s Vision CT became the first FDA-approved clinical CT system 3D specimen radiography, according to the company.
The Vision CT is designed to provide easily navigable, true 3D data and rendering of the specimen, in addition to immediate traditional orthogonal images, said Donogh O’Driscoll, Faxitron’s chief operating officer. As a result, it enables enhanced margin assessment and drives the potential for improved surgical outcomes.
The compact mobile system is used in the operating room for quick excised specimen margin assessment during surgery. Wait times when sending a specimen to radiology can be 15 to 30 minutes during surgery, O’Driscoll said.
O’Driscoll said the company “brought specimens into the 3D world” with the launch of the VisionCT. The system can reconstruct the whole specimen volume — up to 512 frames — in under four minutes in addition to providing traditional orthogonal images in under 30 seconds, which means surgeons can move through the specimen in slices, with the goal of reducing re-incision rates and callbacks, according to O’Driscoll.
“They can interrogate the specimen to a far deeper level,” O’Driscoll said. “It’s far better to provide as much detail as possible and put that in the hands of physicians to make the ultimate call for better patient outcomes.
“Abnormalities in breast tissue are often caught at earlier stages now, thanks to advancements in imaging and breast screening. So there is a parallel need for ongoing advancements when assessing and confirming margin status during surgery,” O’Driscoll said. “With VisionCT, providers can now obtain a clearer, more detailed view of breast specimen margin status than ever before, helping to achieve better surgical outcomes. For decades we’ve been committed to pioneering new technologies that contribute to a higher standard of care for patients and we are proud that our legacy continues with the launch of this innovative new system.”
Fujifilm
Fujifilm introduced two products last year. In January 2017, it upgraded the ASPIRE Cristalle, introduced in 2013, to include digital breast tomosynthesis, and at the end of 2017, the company released mobile tomosynthesis.
“Even for women who do have insurance, only 68 percent of them have had a recent mammogram,” said Susan Crennan, women’s health product marketing manager for FUJIFILM Medical Systems U.S.A., Inc. “We want to make sure all women have access to the latest technologies.”
The company works with a range of mobile imaging vendors for the exams.
The ASPIRE Cristalle has an amorphous selenium detector with Hexagonal Close Pattern (HCP) capture circuitry design, which Crennan said outperforms traditional pixel arrays with a 20 percent increase in detector sensitivity. With 50 microns of resolution, the tomosynthesis exposure time on the ASPIRE Cristalle is four seconds or less, meaning a low likelihood of patient movement, Crennan said. There is also a 15-second interval between subsequent images.
There’s no need to wait for the machine and technologist,” Crennan said. “Other systems tend to have longer intervals — up to 40 seconds — between exposures.”
This year, the company released Dynamic Visualization II for Mammography (DVIIm). The second generation of the software offers intelligent auto recognition of breast tissue, implants and structural characteristics to intelligently adapt image contrast and density, for enhanced diagnostic visibility, according to the company. DVIIm also provides improved contrast and density stability throughout the exposure region and achieves improved visibility across a wide range of breast compositions.
Galen MRI
Galen MRI is currently going through the FDA approval process for its new 1.5-tesla breast MR system, called Emma.
David Tse, the board chairman for Galen MRI, said the abbreviated protocols for Emma reduce the scan time from 45 minutes to 15 minutes. This, in turn, lowers the cost of the scan and helps reduce the cost to around $600 per scan. The AB protocol was tested on 1,500 women at the Memorial Sloan Kettering Imaging Center in New York City.
The system comes with three sizes of breast coils, and the company is in the process of developing a system to reduce noise level. A cloud system, for educational use, will allow the DICOM images to be shared with privacy protection.
There are also machine learning protocols to further reduce the time between the preparatory scan and the proper scan, and two AI systems. The first, called Scan Assistant, scans the images to enhance radiologists' diagnosis, while Report Assistant generates the initial scan report, to facilitate further reporting.
Galen also plans to work with other big data teams, such as Watson's big data team on breast cancer, to further advance the knowledge of breast cancer detection and containment, Tse said.
“With its dedicated coil and AB protocol, Emma will simultaneously help advance early breast cancer detection and lower the cost of breast MR,” Tse said.
GE Healthcare
After the March 2017 debut of its Senographe Pristina 3D Mammography System, GE Healthcare launched the Pristina Dueta, a patient-assisted compression device that the company says can help make the exam less painful.
After the technologist positions the patient, they then give the device to the patient, who can do a final adjustment.
It reduces the patient’s perceived pain levels,” explained Agnes Berzsenyi, president and CEO of GE Healthcare women’s health. They feel empowered and they can be active during the exam. If you pinch yourself, versus someone else pinching you, it’s a big difference.”
In patient studies of the device in Italy and France, four in five of the 160 patients who used the Pristina Dueta said they felt more comfortable during the exam.
In a clinical evaluation of the Dueta used with 30 patients at the Christine E. Lynn Women's Health & Wellness Institute at the Boca Raton Regional Hospital, as part of the FDA submission, medical director Dr. Kathy Schilling, found that patient-assisted compression, compared to compression solely applied by the technologist, produced images of similar quality and didn’t significantly increase the time of the exam.
The technologist sets the compression to the minimum required threshold. The patient can increase compression or they can decrease only once.
Schilling also found that women who were in control of their own compression actually compressed the breast 45 percent more than the technologist did.
In November 2017, GE released the SenoBright HD clinical application. The Contrast Enhanced Spectral Mammography (CESM) exam, available on the Senographe Pristina, can be used as an alternative to breast MR for patients at high risk for breast cancer or who have inconclusive mammogram or ultrasound results.
The IV iodine injection is performed in same room as the mammogram. The exam takes less than seven minutes and the images are available immediately for a radiologist to review.
“It’s a great fit for a facility without access to MR onsite,” Berzsenyi said.
On the software side, GE released Mammo Insights in November 2017. The multivendor software analyzes data using algorithms to gain insight into different types of exams. It looks at dose by breast thickness and composition so facilities can adjust imaging techniques and can compare dose among multiple sites.
“There is a need for understanding of dose,” Berzsenyi said. “We’re starting to see trends where patients are demanding it and they want to know.”
Hologic
Hologic has released several products in the breast imaging space in recent months, with a focus on image quality, improved patient experience and workflow.
The company's 3Dimensions Mammography System, which was FDA cleared in June 2017, has a proprietary feature called the SmartCurve breast stabilization system, which was FDA cleared separately this past March.
The paddle, which mimics the shape of the breast, is designed to deliver a more comfortable experience for the patient, said Tracy Accardi, Hologic's global vice president of research and development, breast and skeletal health solutions.
"The SmartCurve breast stabilization system delivers a more comfortable mammogram without sacrificing image quality," Accardi said.
SmartCurve is also available for previous generations of Hologic mammography systems.
The 3Dimensions system also features Clarity HD high-resolution 3D imaging, which Accardi said provides the highest resolution 3D images in the industry, and can detect subtle lesions, regardless of breast size and density, to pinpoint cancer earlier. Additionally, the Genius 3D Mammography exam, which is available on the 3Dimensions system, reduces callbacks by up to 40 percent compared to 2D imaging.
"The 3Dimensions system is the next generation of 3D imaging," said Accardi of the technology.
In August, Hologic released its Brevera breast biopsy system, with CorLumina imaging technology, in the U.S. It provides real-time imaging and automated post-biopsy specimen handling, negating the need to take samples out to another room for further review.
"The system makes sure you are getting the cancerous tissue you intended to get," Accardi said. "Every eight seconds you can obtain a biopsy sample and view its X-ray. The result is a much quicker workflow for the radiologist, and a faster procedure for the patient."
In May of this year, Hologic released a new portable breast ultrasound system called the Viera portable breast ultrasound system.
The transducer and processing are all handheld and images are transmitted wirelessly to a mobile device or a PACS system.
"The system is the perfect tool for a breast surgeon about to do a biopsy, and makes sure they're looking at the right place in the woman's breast," Accardi said. "They don't have to find and cart in a larger ultrasound system from another room."
The device can also negate the need for wire localization, which would be a win for both a workflow and patient experience perspective, Accardi said.
"Traditionally, portable ultrasound systems have a reputation for compromising on image quality," Accardi said. "However, we've optimized this system to have the best possible image quality and physician workflow. When you're done with the Viera system, you drop it right in your pocket."
In March, Philips announced a partnership with Hologic to combine Hologic's mammography technologies and Philips' portfolio of ultrasound, MR, CT, and X-ray systems, advanced informatics and range of services, including maintenance, upgrades, training and operational performance management services.
In a statement announcing the partnership, Philips said it will allow hospitals easier access to integrated suites of diagnostic imaging modalities, advanced informatics and services for comprehensive breast screening and diagnosis.
iCAD
In March 2017, iCAD received FDA approval for PowerLook Tomo Detection, a computer-aided detection solution for breast tomosynthesis that is built on deep learning technology.
The solution is available for use worldwide with GE Healthcare breast tomosynthesis, though the company received CE Mark approval for a new version of the product for use in Europe with Hologic and Siemens systems, as well as those from GE, and looks to having a solution available for other vendors in the future.
Part of the PowerLook Breast Health Solutions platform, PowerLook Tomo Detection identifies suspicious areas in breast tomosynthesis images.
Rodney Hawkins, vice president of marketing for iCAD, said a reader study showed a 29.2 percent reduction in reading time without impacting clinical performance.
Hawkins calls the deep learning technology similar to traditional computer-aided detection methods, “but more advanced.”
Traditional CAD technology “is a little more manual in how the algorithm is taught to look for cancer,” Hawkins said. “A researcher basically guides the algorithm to find cancer. Deep learning is a more advanced subset of machine learning. The computer learns the necessary features itself. We train it on actual cancer cases and it learns on its own what the characteristic features of cancer are. It’s a much more accurate technology. … Similar to 2D CAD, it provides them with a level of confidence, another set of eyes. It’s a lot easier in tomo to miss things because there’s so much information. We’ve heard customers say they’re less fatigued.”
The new product that is currently available in Europe detects soft tissue density and calcifications and provides more information on the certainty of findings, or “how confident the algorithm is,” with a confidence score from zero to 100, Hawkins said.
Ikonopedia
Last year, Ikonopedia released the latest version of its Ikonopedia Breast Reporting and Tracking software.
The new version incorporates a tablet-based questionnaire with the only web-based version of the Tyrer-Cuzick Breast Cancer risk assessment tool utilizing Tyrer-Cuzick version 8, which incorporates density, according to Emily Crane, Ikonopedia's chief executive officer.
Ikonopedia's software also employs what Crane said is the only "closed loop" tracking at a lesion-specific level, which prevents any finding from being lost to follow-up.
In December 2017, the company also released a full EQUIP module to support the new image quality requirements from the FDA.
The EQUIP software gives imaging departments the ability to report, track and address poor image quality at the point of read and also creates randomized audits for review. It then creates all necessary outcome reports.
"They never have to worry about doing a manual audit," Crane said. "We automate all necessary audits for them and they can be printed at the touch of one button."
In 2017 Ikonopedia also released Patient Canvas, which gives technologists a visual tool to markup things seen or felt on the outside of the breast — scars, moles, lumps, or pain — so the radiologist might investigate areas with external issues. Patient Canvas also alerts the radiologists when they open the case to any findings on the Patient Canvas, which Crane said enhances technologist and radiologist communication.
IntraMedical Imaging
IntraMedical Imaging recently received CE Mark approval for the Node Seeker 2000, a wireless gamma probe with a tablet-based control unit that can simultaneously find primary tumors based on Iodine 125 (125I) radioactive seed localization and in the sentinel lymph node.
Farhad Daghighian, the chief executive officer of IntraMedical Imaging, said the system, released last year, gives an accurate estimate of the depth of the seed and corrects for the interference of technetium gamma rays while searching for the seed.
“The battery-operated tablet is made for medical use, and by wrapping it in a sterilized jacket, it can be placed on top of the patient in front of the surgeon so the surgeon would not need the nurses to adjust the sound or other parameters,” Daghighian said.
The market for the Node Seeker 2000 is general surgeons, surgical oncologists and nuclear medicine departments.
The Node Seeker 2000 can control gamma ray detection probes and beta probes that IntraMedical manufactures for detection of positron emitting tracers. These PET probes are being used for intraoperative detection of tumors filled by F-18 FDG.
Koning Corporation
Koning Corporation’s Koning Breast CT (KBCT) system was FDA approved in 2015; a redesign reducing the footprint and room size, with input power similar to that of a conventional mammography system, got an FDA nod last November.
The Koning KBCT system removes painful breast compression and is a multi-application device FDA-cleared for both the diagnostic workup as well as a 3D guided biopsy, said David Georges, president of the company’s USA division. It fits into a 10-foot-by-14-foot room, which is similar to rooms designed for stereotactic biopsy.
“Prior to the redesign, the KBCT required the same installation space and input power as a full-body CT,” Georges said. “Doctors could actually proceed right to the 3D guided biopsy after the diagnostic workup if that’s what they choose to do.”
The company claims that with breast compression eliminated, the exam is much more comfortable for the patient.
“We’ve not had a woman out of the thousands of patients we’ve imaged say they’d prefer a mammogram,” Georges said.
With floor space a premium in most facilities, the ability to perform the diagnostic workup and a biopsy in the same room delivers new efficiencies in breast imaging, Georges said.
The technology is based on isotropic imaging of the breast and uses a mammographic X-ray tube and detector to gather true 3D volumetric images in under 10 seconds.
“It means we can visualize areas of interest from any angle equally,” Georges said. “Conventional linear tomography of the breast is a limited sweep angle acquisition and not the way we look at the head, chest and abdomen with whole body CT imaging. Breast tomosynthesis is pseudo 3D, whereas Koning is introducing true volumetric 3D of the breast.
“What physicians are telling us [is that] they finally have images that are free from distortion from compression,” Georges said. “Tissue is free from overlapping structures, a known cause of missed cancers in mammography. Until you separate structures, you will never have a clear view of what is cancer and what is not cancer.”
Georges said the dose is in the same range as dose from a diagnostic mammogram and equal to or less than digital breast tomosynthesis for a diagnostic workup. As with all imaging, dose is dependent on the size and thickness of the breast.
“The next challenge for us is to design a smaller device that will deliver low-dose screening exams integrating our true isotropic 3D acquisition process to obtain clear unobstructed images of early stage breast cancers before they spread,” Georges said. “We’re very close to achieving this goal.”
The company has 12 installations, two of which are the new design — one in Knoxville, Tennessee and one in Bangkok, Thailand.
There is currently a study of contrast-enhanced breast CT at the University of Rochester, where research for the device originally began. “Similar to whole body CT with and without contrast, we anticipate significant advantages by applying contrast-enhanced breast CT imaging where applicable,” Georges said.
Kubtec
Kubtec’s MOZART System, an intraoperative tomosynthesis scanner designed to be used during lumpectomies, received an upgrade recently with the implementation of voice control and augmented intelligence.
The voice control feature allows surgeons to operate the system using simple commands — generated by focus groups of surgeons — without breaking the sterile field.
“They can give commands to the instrument itself,” said John Leach, vice president of marketing for Kubtec. "They can run any 3-D scans, zooms or highlight areas of interest."
Surgeons have said they appreciate the fact that they don’t have to break scrub or direct OR staff to use the machine.
“It doesn’t talk back,” Leach joked.
The new augmented intelligence automatically identifies the lesion in the image and can show the surgeon whether they’ve removed the entire tumor.
The features are available as a separate package for the MOZART called the AI package, released in May at the American Society of Breast Surgeons annual meeting in Orlando. It is also packaged with new systems.
"We believe the space used in the OR is too valuable to be used to produce just an image,” Leach said. “Our focus is on enhancing the physician's ability to get the information they need from the image in order to make rapid, informed intraoperative decisions that benefit patient outcomes.”
QView Medical
In December, QView Medical received FDA PMA approval for its QVCAD solution for use with the GE Invenia 3D ABUS automated breast ultrasound system. In March 2017, it received the CE Mark for use of the product with Siemens as well as GE.
The AI product is used for screening asymptomatic patients with dense breasts after a negative mammogram.
CAD for mammography has become the standard of care, so it was natural for the company to develop a similar product, using deep learning for automated breast ultrasound exams, said Bob Foley, vice president of regulatory affairs and marketing strategy for QView Medical. Each ABUS exam leads to images with 2,000 slices, with a read time of five to eight minutes.
“We recognized there was a need to be able to read more productively,” Foley said.
Foley said the QVCAD is able to reduce reading time to approximately two minutes without compromising diagnostic accuracy.
Screenpoint Medical
This Netherlands-based company recently released Transpara, an AI product that helps radiologists read mammograms and digital breast tomosynthesis, for use in Europe.
Nico Karssemeijer, the chief executive officer of Screenpoint Medical, said Transpara is different from CAD in that it provides more than a guide to the images.
“CAD systems are very limited because they only point out regions for radiologists to look at,” Karssemeijer said. “Our system interprets abnormalities and provides a cancer suspiciousness score for the whole mammogram that can be used as a second opinion. With the current release, we are reading mammograms as good as experienced radiologists.”
Karssemeijer stressed that the product is not intended to replace radiologists, noting that in the European market, two radiologists are required to read a mammogram.
“This is intended to partly replace one of the two,” Karssemeijer said. “There are some countries where there is a big shortage of radiologists. It’s only going to become more difficult with breast tomosynthesis. This is one of the big problems we have to solve before tomo is used for breast screening programs in Europe.”
The company is in the process of getting FDA clearance for Transpara and is currently doing clinical studies in the U.S.
Siemens Healthineers
In March, Siemens Healthineers received FDA clearance for its MAMMOMAT Revelation, which was shown at RSNA 2017.
The premium mammography system features an integrated specimen imaging tool called InSpect, as well as a 3D tomo breast biopsy solution, the ability to perform contrast-enhanced mammography, and automated breast density measurement at the point of care.
“This option can save time, reduce costs and minimize the anxiety of being called back, especially where a woman might have to wait for an MR,” said Pam Cumming, senior director of product marketing for women's health at Siemens Healthineers North America. “It does give another nice option for people who don’t have easy access to MR.”
The MAMMOMAT Revelation also builds on the previous generation system, the MAMMOMAT Inspiration, with a wide, 50-degree field of view that allows better separation of overlapping breast tissue, resulting in improved visualization of subtle abnormalities, Cumming said.
“Overlapping tissue is a problem in mammography,” said Cumming, who noted that the depth resolution is 3.5 times higher than that of their competitors’ systems.
Cumming also noted that the wide angle might one day allow for reduced compression.
“Clinical data is saying you might not need to do as much compression,” Cumming said. “It’s the power of the wide angle that’s creating the basis for conversation and what might be possible.”
The MAMMOMAT Revelation also performs automated breast density measurement during the mammogram, instead of after the patient has left the imaging facility.
Volpara
Volpara recently released a new software feature that provides feedback on how well the technologist did positioning the patient, by assigning mammographic studies into perfect, good, moderate and inadequate categories.
Training videos, which feature Louise Miller, co-founder of Mammography Educators, are embedded in the company's VolparaEnterprise software and will be based on exam statistics.
The software performs an automated assessment of positioning on every mammogram and will note, for example, if the technologist is not getting inframammary folds into the image, said Julian Marshall, Volpara’s chief product officer.
Since the feature is a quality control device, it doesn’t require FDA clearance.
The software, with cost depending on how big the facility is and how many mammograms it does, is designed to reduce the number of technical recalls, which facilities don’t receive reimbursement for.
“Our key products are being adopted by large health systems because they see it increasing quality across the system,” Marshall said.