December New Product Showcase
December 17, 2016
Image processing
RamSoft has introduced a new remote rendering solution. Through the technology, images are
streamed much like an online webinar, eliminating the need to download mass amounts of data to your computer. This process significantly improves efficiency and reduces the response time from
click to display. Remote rendering also reduces costs and hardware requirements, eliminates the need to create and manage pre-caching rules, and operates with zero-footprint architecture, so you can access data on any browser-capable device via RamSoft’s zero-footprint viewer, RapidResults™.
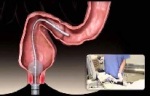
Single-use, self-propelled colonoscope
GI View Ltd., developer of advanced GI screening systems, announced that it has received FDA 510(k) clearance for the new Aer-O-Scope Colonoscope System, a disposable, self-propelled, joystick-controlled, easy-to-use colonoscope system, now with therapeutic access. The new system has two working channels that enable therapeutic access using standard tools, such as snares and forceps, to take biopsies or perform polypectomies. The Aer-O-Scope is the first colonoscope to provide a 360° omni-directional visualization of the colon to detect polyps behind folds.
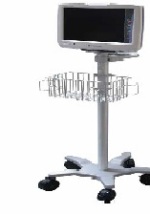
Bedside monitor
Nihon Kohden has launched its BSM-3500, the latest advancement in its line of bedside monitors. Designed to meet the precise needs of ambulatory surgery and specialty centers, the BSM-3500 comes fully optimized with all features, both premium and standard, ready to use at a moment’s notice, bringing among the highest standards of care to low-acuity settings. In addition to a range of standard monitoring features, the BSM-3500 offers a number of critical capabilities that include the ability to spot real-time mini trends for early detection of vital sign variability during outpatient procedures. In addition, the new monitor works with Nihon Kohden’s proprietary cap-ONE Mainstream CO2 Sensor Kit, which is the world’s first wearable mainstream CO2 sensor for non-intubated patients. As with all Nihon Kohden monitoring systems, the BSM-3500 is also designed to seamlessly integrate with electronic medical records systems.
Nodule detection application
Riverain Technologies announced FDA clearance of ClearRead CT, a new nodule detection application. ClearRead CT is the only FDA-cleared device to support concurrent reading, allowing for faster reading with proven superior automatic nodule detection performance for all primary nodule types, including solid, sub-solid and ground glass nodules. ClearRead CT, powered by acquisition normalization technology, provides an enterprise-ready solution for the entire health care network. The technology processes CT scans from a wide range of manufacturers and acquisition protocols, and quickly installs into the clinical environment without the need for new hardware or customized tuning to specific devices or protocols.
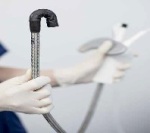
Visualization and diagnostic endoscopy device
The FDA has given 510(k) clearance for the invendoscopy E200 system. The system, developed by invendo medical GmbH, includes the invendoscope SC200, the first and only sterile, single-use colonoscope. The invendoscopy SC200 is a simple, safe and effective solution for clinical and hygienic challenges, ensuring that a new colonoscope is always ready for physicians to use and that each patient receives his or her own device. The invendoscopy technology provides robotic assistance for tip control and a new design to offer gastroenterologists greater control and enhanced comfort while performing procedures.
RamSoft has introduced a new remote rendering solution. Through the technology, images are
streamed much like an online webinar, eliminating the need to download mass amounts of data to your computer. This process significantly improves efficiency and reduces the response time from
click to display. Remote rendering also reduces costs and hardware requirements, eliminates the need to create and manage pre-caching rules, and operates with zero-footprint architecture, so you can access data on any browser-capable device via RamSoft’s zero-footprint viewer, RapidResults™.
Single-use, self-propelled colonoscope
GI View Ltd., developer of advanced GI screening systems, announced that it has received FDA 510(k) clearance for the new Aer-O-Scope Colonoscope System, a disposable, self-propelled, joystick-controlled, easy-to-use colonoscope system, now with therapeutic access. The new system has two working channels that enable therapeutic access using standard tools, such as snares and forceps, to take biopsies or perform polypectomies. The Aer-O-Scope is the first colonoscope to provide a 360° omni-directional visualization of the colon to detect polyps behind folds.
Bedside monitor
Nihon Kohden has launched its BSM-3500, the latest advancement in its line of bedside monitors. Designed to meet the precise needs of ambulatory surgery and specialty centers, the BSM-3500 comes fully optimized with all features, both premium and standard, ready to use at a moment’s notice, bringing among the highest standards of care to low-acuity settings. In addition to a range of standard monitoring features, the BSM-3500 offers a number of critical capabilities that include the ability to spot real-time mini trends for early detection of vital sign variability during outpatient procedures. In addition, the new monitor works with Nihon Kohden’s proprietary cap-ONE Mainstream CO2 Sensor Kit, which is the world’s first wearable mainstream CO2 sensor for non-intubated patients. As with all Nihon Kohden monitoring systems, the BSM-3500 is also designed to seamlessly integrate with electronic medical records systems.
Nodule detection application
Riverain Technologies announced FDA clearance of ClearRead CT, a new nodule detection application. ClearRead CT is the only FDA-cleared device to support concurrent reading, allowing for faster reading with proven superior automatic nodule detection performance for all primary nodule types, including solid, sub-solid and ground glass nodules. ClearRead CT, powered by acquisition normalization technology, provides an enterprise-ready solution for the entire health care network. The technology processes CT scans from a wide range of manufacturers and acquisition protocols, and quickly installs into the clinical environment without the need for new hardware or customized tuning to specific devices or protocols.
Visualization and diagnostic endoscopy device
The FDA has given 510(k) clearance for the invendoscopy E200 system. The system, developed by invendo medical GmbH, includes the invendoscope SC200, the first and only sterile, single-use colonoscope. The invendoscopy SC200 is a simple, safe and effective solution for clinical and hygienic challenges, ensuring that a new colonoscope is always ready for physicians to use and that each patient receives his or her own device. The invendoscopy technology provides robotic assistance for tip control and a new design to offer gastroenterologists greater control and enhanced comfort while performing procedures.