December New Product Showcase
December 28, 2015
by Sean Ruck, Contributing Editor
Healthcare Records
To provide patients with a one-stop destination for all of their medical records, Medfusion — a patient engagement platform dedicated to facilitating the relationship between doctors and patients — has developed a consumer mobile application — Medfusion Plus.
Via a download on the App Store and Google Play, iOS and Android device users can now aggregate and access their healthcare records onto their mobile devices. The information is pulled via Continuity of Care documents (CCD) from the patient portals and includes laboratory results, immunizations, medication lists, allergies, as well as appointments.
Medfusion Plus, which also provides an application programming interface and is available on the Apple Watch, will continue to evolve with a significant roadmap ahead for the developers.
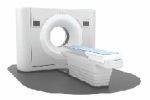
Diagnostic Suite for IQon Spectral CT
Royal Philips announced that its IQon Spectral CT now has increased potential thanks to a new diagnostic suite approved for the U.S. market.
By providing spectral capabilities within traditional CT applications, the Spectral Diagnostic Suite may unlock a new level of flexibility and clinical utility for the workhorse imaging modality. It may also reduce repeat imaging, as incidental findings can be explored in greater depth with the spectral data sets.
The visual and analytical benefits of the Spectral Diagnostic Suite can be tapped into from a variety of different settings. According to a statement from Philips, the spectral data can be viewed from a reading room, a PACS system, or a remote location.
The IQon Spectral CT system itself received FDA approval in November of 2014.
High Intensity Focused Ultrasound Prostate Ablation Device
SonaCare Medical, LLC announced that it received de novo clearance from the U.S. Food and Drug Administration (FDA) to market the Sonablate® 450 in the U.S. for the ablation of prostate tissue. Sonablate® is the first High Intensity Therapeutic Ultrasound (HITU) device to receive FDA regulatory authorization for prostate tissue ablation.
Patient Monitoring System
Qualcomm Incorporated announced that Capsule, a wholly owned subsidiary of Qualcomm Life, Inc., has received FDA 510(k) clearance of SmartLinx Vitals Plus, a patient monitoring system that integrates vital signs monitoring and clinical documentation into one scalable platform. SmartLinx Vitals Plus, part of Capsule’s SmartLinx Medical Device Information System™, is a powerful alternative to traditional low acuity monitors. It provides new functionality in a single platform that allows hospitals and other health care facilities to streamline user authentication, patient identification, vitals measurement and clinical documentation by integrating vital signs modules and components directly to the SmartLinx NeuronTM 2 mobile clinical computer.
Patient Relationship Management Solution
Salesforce recently launched its first industry product built specifically for health care. Salesforce Health Cloud is a cloud-based, patient relationship management solution. It helps providers build stronger, one-to-one relationships; make smarter care decisions; and connect with their patients anywhere, on any device. It features a Today screen with alerts to caregivers on timely patient issues; Patient Profiles and Timeline views that provide a central view of a patient’s history and easy-to-read clinical data for providers; Private Communities that allow care coordinators to collaborate and assign tasks across a caregiver’s network; and Salesforce Shield, a set of built-in features that allow providers to manage, audit and archive patient data.
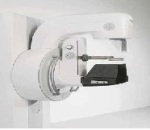
New Full-field Digital Mammography System
Siemens Healthcare recently announced the FDA has cleared the Mammomat Fusion mammography system.The system features a new generation cesium-iodide detector — an innovative, layered configuration of the photo diodes within the detector that enables more efficient utilization of the radiation dose. The result: high image quality at a patient dose that is at or below the range of other full-field digital mammography systems, with an even lower dose delivered in cases where the patient’s breast thickness exceeds 50 mm. And the system’s large image matrix of 23x30 cm makes the Mammomat Fusion the right choice for screening various breast sizes.
To provide patients with a one-stop destination for all of their medical records, Medfusion — a patient engagement platform dedicated to facilitating the relationship between doctors and patients — has developed a consumer mobile application — Medfusion Plus.
Via a download on the App Store and Google Play, iOS and Android device users can now aggregate and access their healthcare records onto their mobile devices. The information is pulled via Continuity of Care documents (CCD) from the patient portals and includes laboratory results, immunizations, medication lists, allergies, as well as appointments.
Medfusion Plus, which also provides an application programming interface and is available on the Apple Watch, will continue to evolve with a significant roadmap ahead for the developers.
Diagnostic Suite for IQon Spectral CT
Royal Philips announced that its IQon Spectral CT now has increased potential thanks to a new diagnostic suite approved for the U.S. market.
By providing spectral capabilities within traditional CT applications, the Spectral Diagnostic Suite may unlock a new level of flexibility and clinical utility for the workhorse imaging modality. It may also reduce repeat imaging, as incidental findings can be explored in greater depth with the spectral data sets.
The visual and analytical benefits of the Spectral Diagnostic Suite can be tapped into from a variety of different settings. According to a statement from Philips, the spectral data can be viewed from a reading room, a PACS system, or a remote location.
The IQon Spectral CT system itself received FDA approval in November of 2014.
High Intensity Focused Ultrasound Prostate Ablation Device
SonaCare Medical, LLC announced that it received de novo clearance from the U.S. Food and Drug Administration (FDA) to market the Sonablate® 450 in the U.S. for the ablation of prostate tissue. Sonablate® is the first High Intensity Therapeutic Ultrasound (HITU) device to receive FDA regulatory authorization for prostate tissue ablation.
Patient Monitoring System
Qualcomm Incorporated announced that Capsule, a wholly owned subsidiary of Qualcomm Life, Inc., has received FDA 510(k) clearance of SmartLinx Vitals Plus, a patient monitoring system that integrates vital signs monitoring and clinical documentation into one scalable platform. SmartLinx Vitals Plus, part of Capsule’s SmartLinx Medical Device Information System™, is a powerful alternative to traditional low acuity monitors. It provides new functionality in a single platform that allows hospitals and other health care facilities to streamline user authentication, patient identification, vitals measurement and clinical documentation by integrating vital signs modules and components directly to the SmartLinx NeuronTM 2 mobile clinical computer.
Patient Relationship Management Solution
Salesforce recently launched its first industry product built specifically for health care. Salesforce Health Cloud is a cloud-based, patient relationship management solution. It helps providers build stronger, one-to-one relationships; make smarter care decisions; and connect with their patients anywhere, on any device. It features a Today screen with alerts to caregivers on timely patient issues; Patient Profiles and Timeline views that provide a central view of a patient’s history and easy-to-read clinical data for providers; Private Communities that allow care coordinators to collaborate and assign tasks across a caregiver’s network; and Salesforce Shield, a set of built-in features that allow providers to manage, audit and archive patient data.
New Full-field Digital Mammography System
Siemens Healthcare recently announced the FDA has cleared the Mammomat Fusion mammography system.The system features a new generation cesium-iodide detector — an innovative, layered configuration of the photo diodes within the detector that enables more efficient utilization of the radiation dose. The result: high image quality at a patient dose that is at or below the range of other full-field digital mammography systems, with an even lower dose delivered in cases where the patient’s breast thickness exceeds 50 mm. And the system’s large image matrix of 23x30 cm makes the Mammomat Fusion the right choice for screening various breast sizes.